



Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Study material related to the title of the above University level Study material of biochemistry All the best for your prospects
Typology: Study notes
1 / 54

This page cannot be seen from the preview
Don't miss anything!















































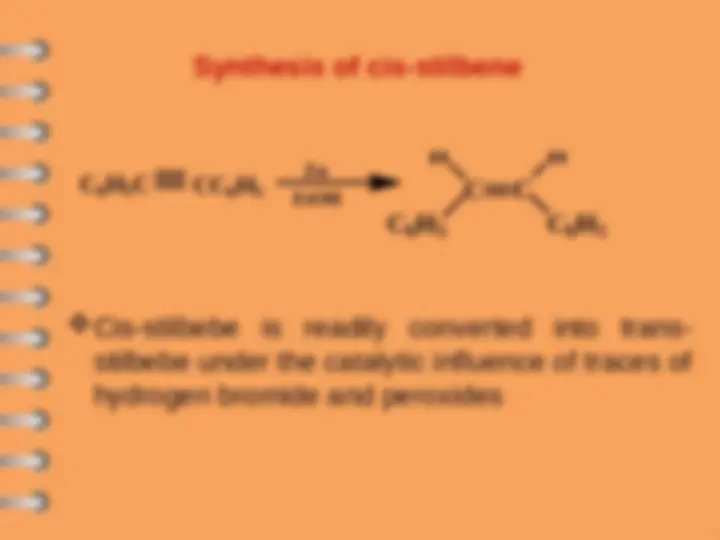
Azulene
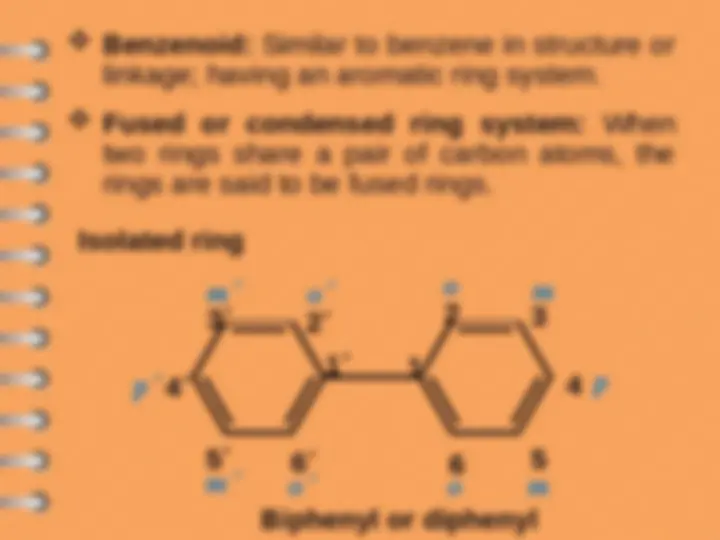
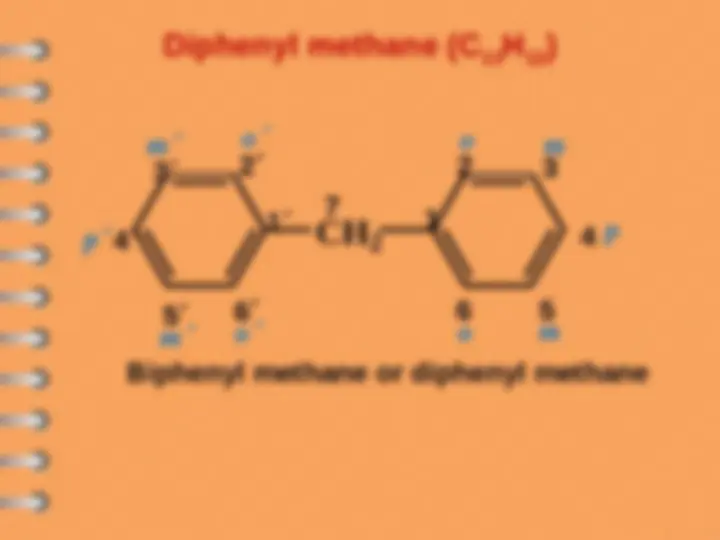
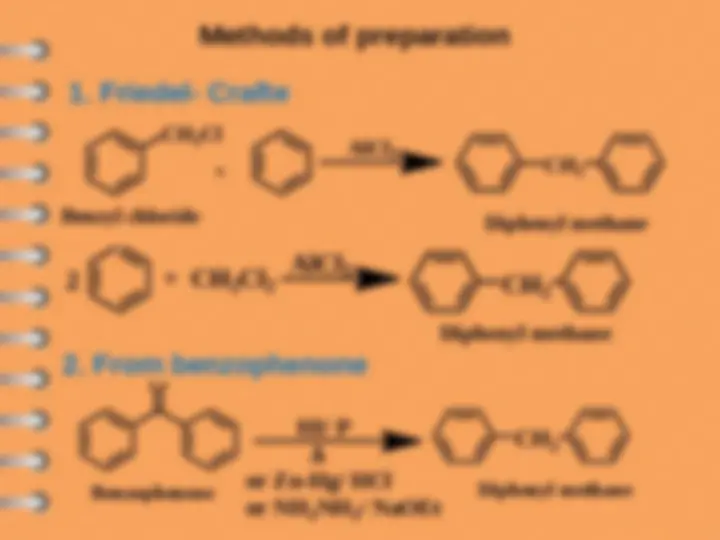
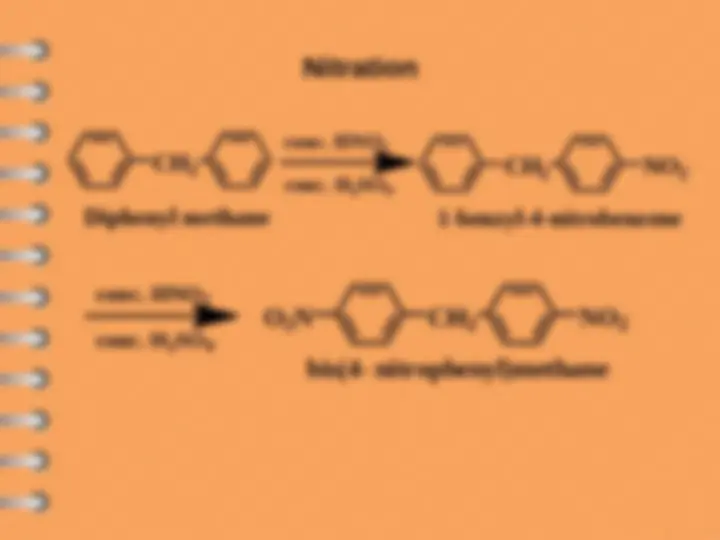
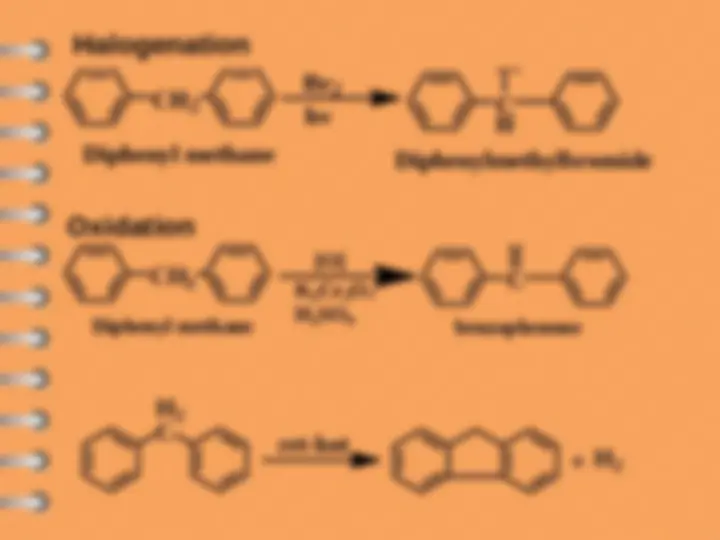
Biphenyl
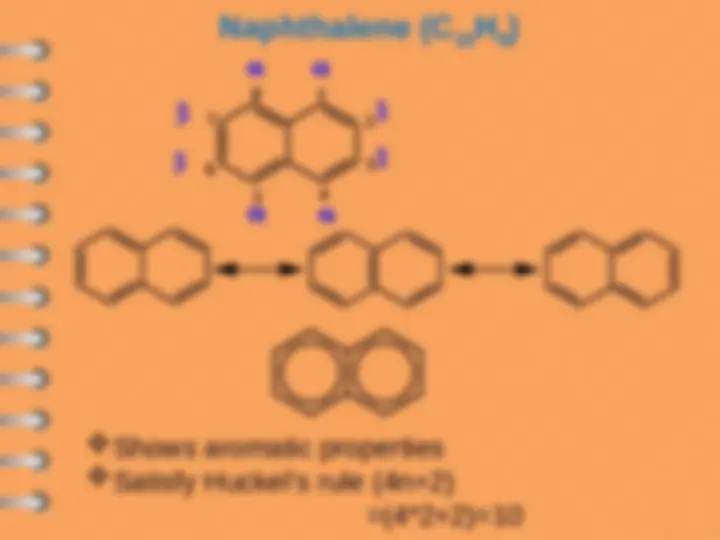
Naphthalene
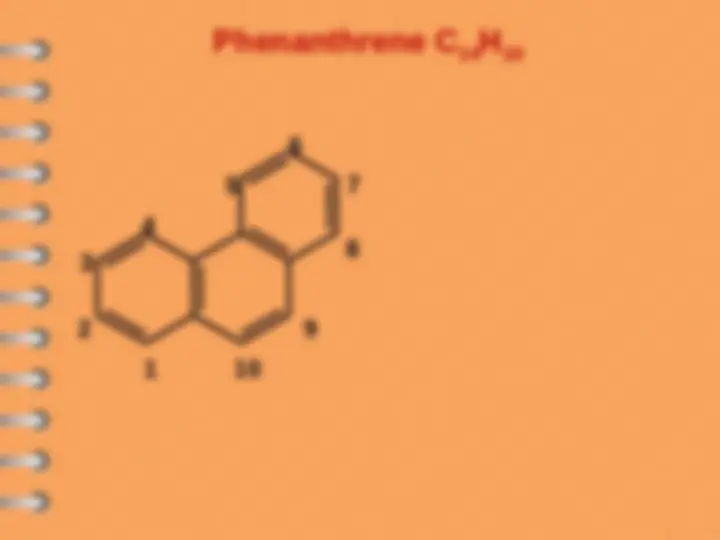
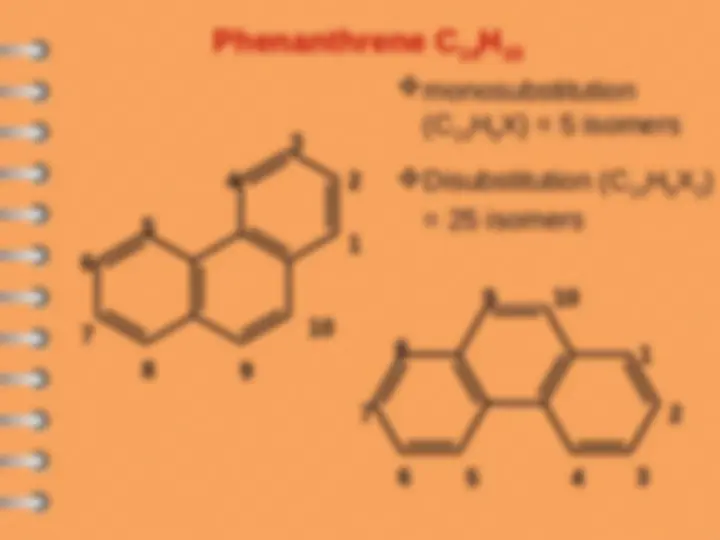
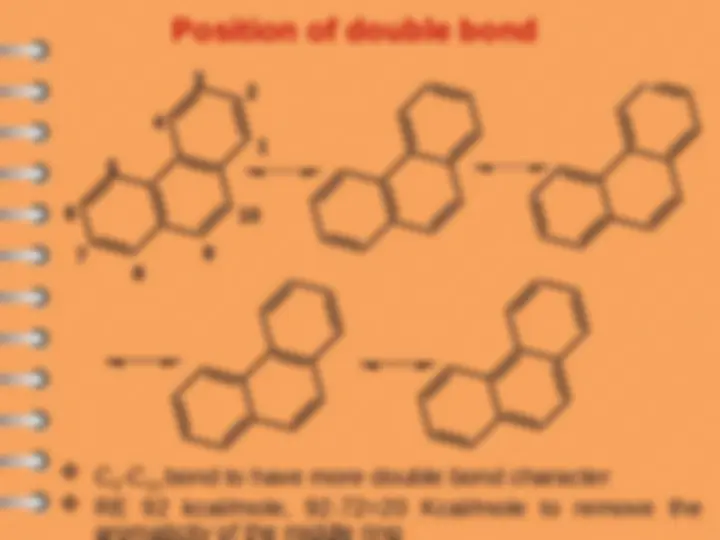
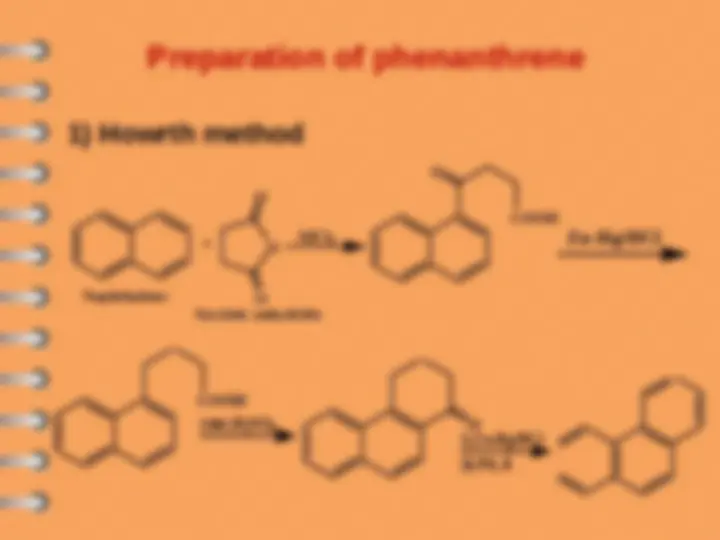
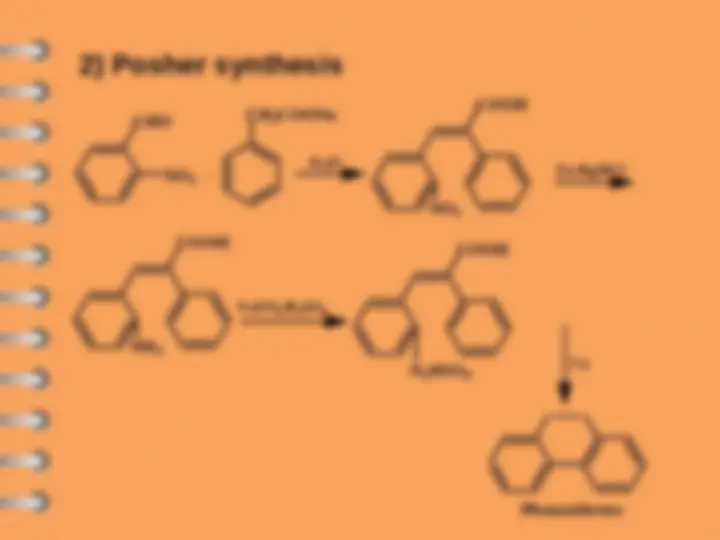
Phenanthrene
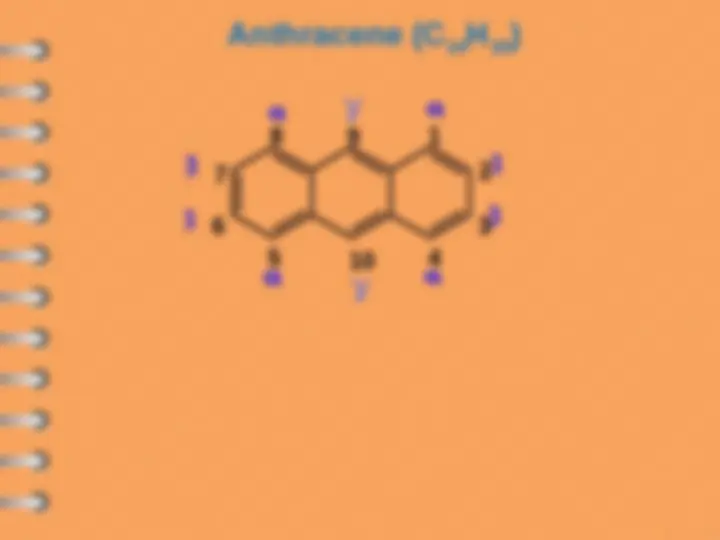
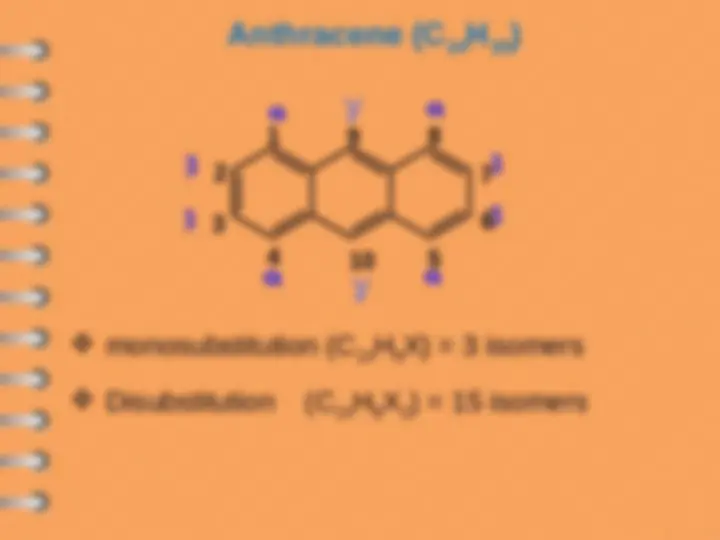
Polynuclear Hydrocarbons
Benzenoid
Non- Benzenoid
Polynuclear aromatic hydrocarbons are
composed by two or more benzene rings
8 1
2
3
4
5
6
7
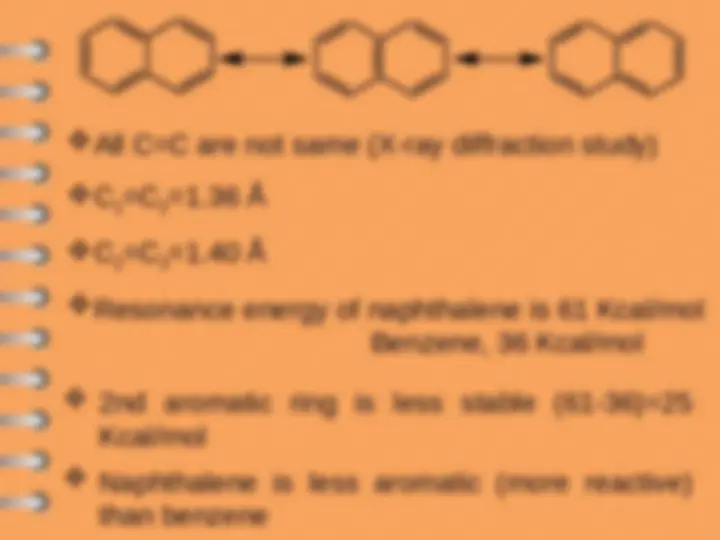
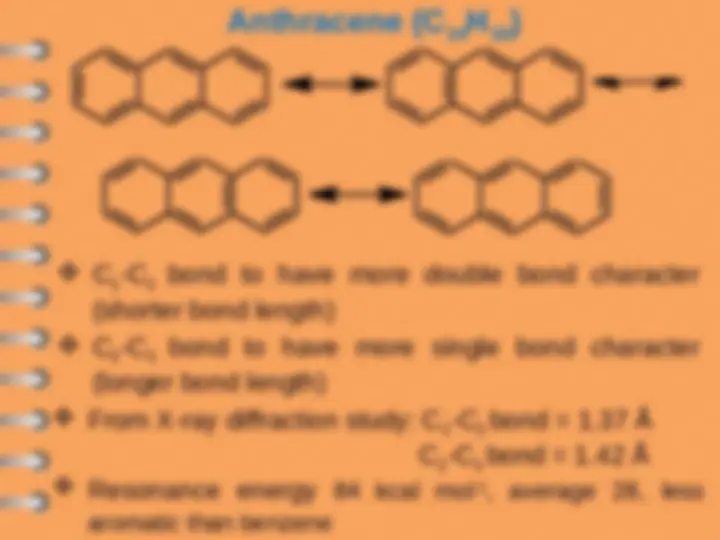
All C=C are not same (X-ray diffraction study)
C
1
=C
2
=1.36 Å
C
2
=C
3
=1.40 Å
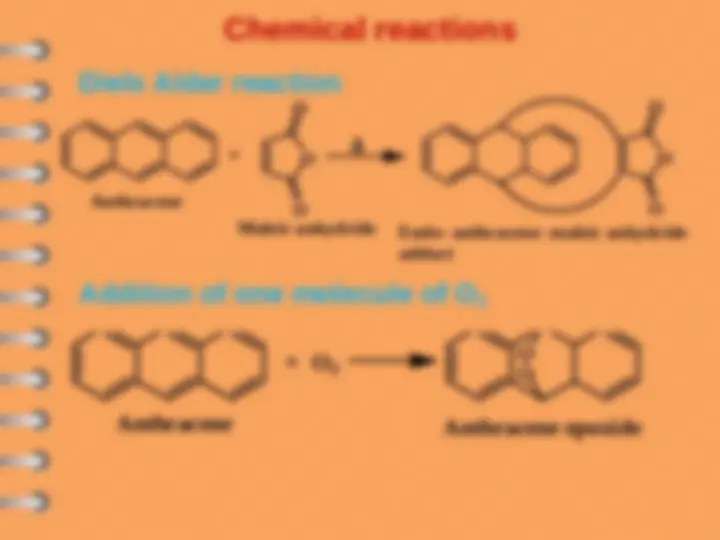
Resonance energy of naphthalene is 61 Kcal/mol
Benzene, 36 Kcal/mol
2nd aromatic ring is less stable (61-36)=
Kcal/mol
Naphthalene is less aromatic (more reactive)
than benzene
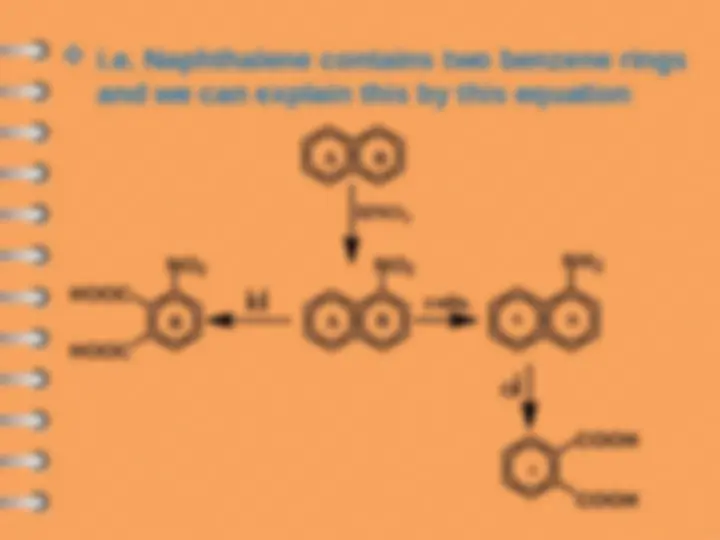
Nitrophthalic acid
2
O
Phthalic acid
COOH
COOH
nitration
redn.
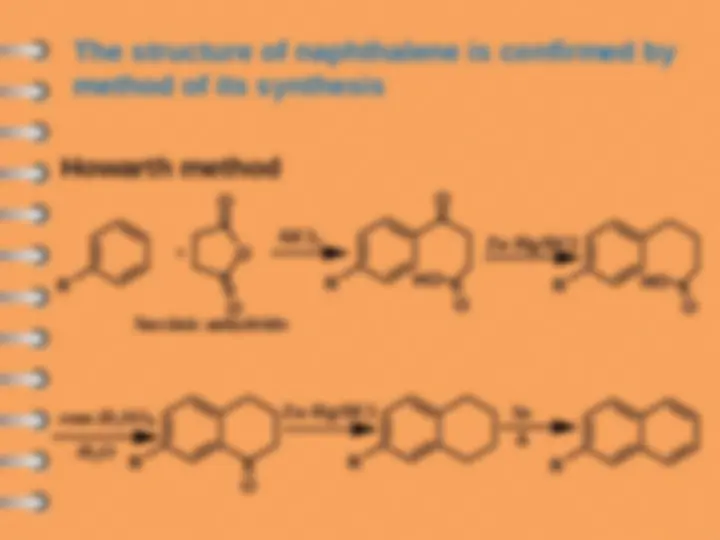
i.e. Naphthalene contains two benzene rings
and we can explain this by this equation
2
2
A B
A
3
2
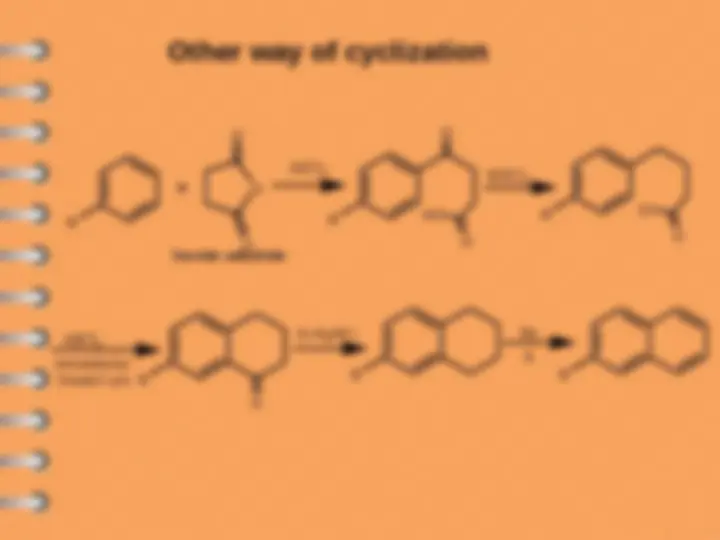
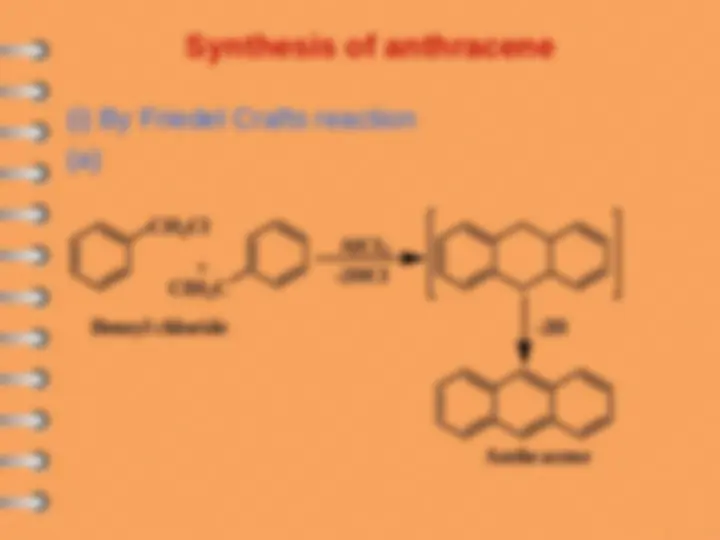
Other way of cyclization
R
O
O
O
AlCl
3
R
O
O
HO
Succinic anhydride
R
O
Cl
SOCl
2
R
O
intramoluclar
AlCl
3
Friedel Craf t
Zn-Hg/HCl
R
Se
R
The reaction occurs if R is o - or p - directing
group such as NH
2
, NHR, OH, OR, R,
halogen.
If R is m - directing group (e.g. NO
2
, CN,
COOH, COCH
3
, SO
3
H) no reaction occur.
The above reaction gives -substituted
naphthalene.
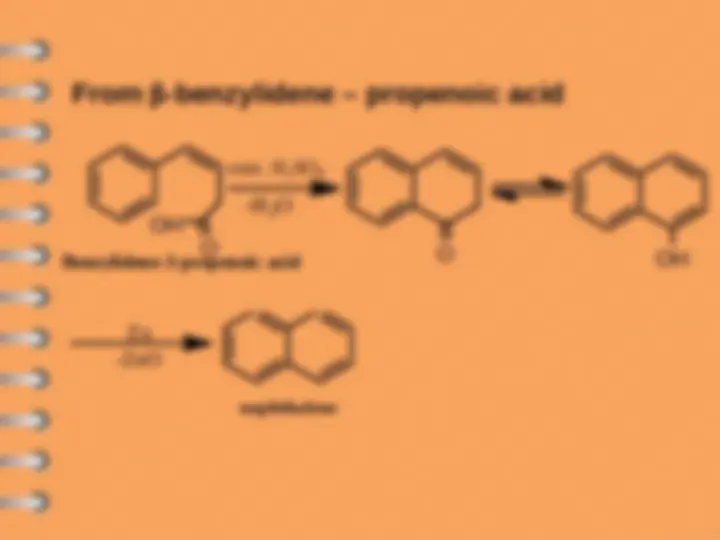
From -benzylidene – propenoic acid
naphthalene
conc. H
2
4
2
Benzylidene- 3 - propenoic acid
2
1 , 4 - dichloro- 1 , 4 - dihydronaphthalene
2
Cl
2
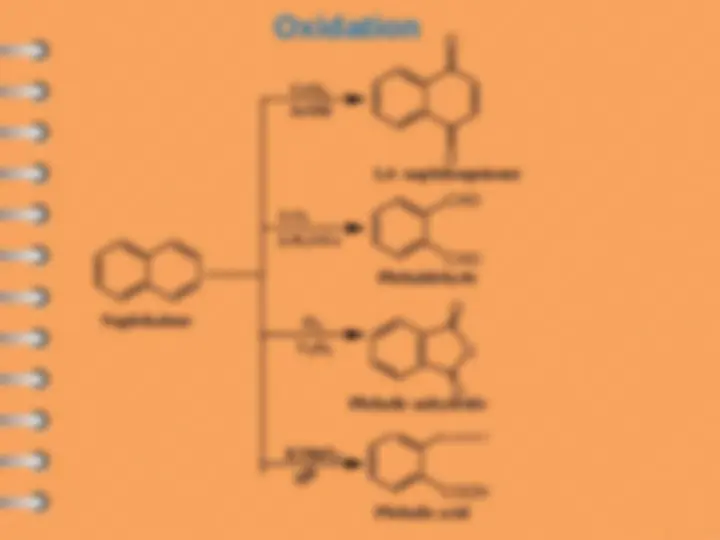
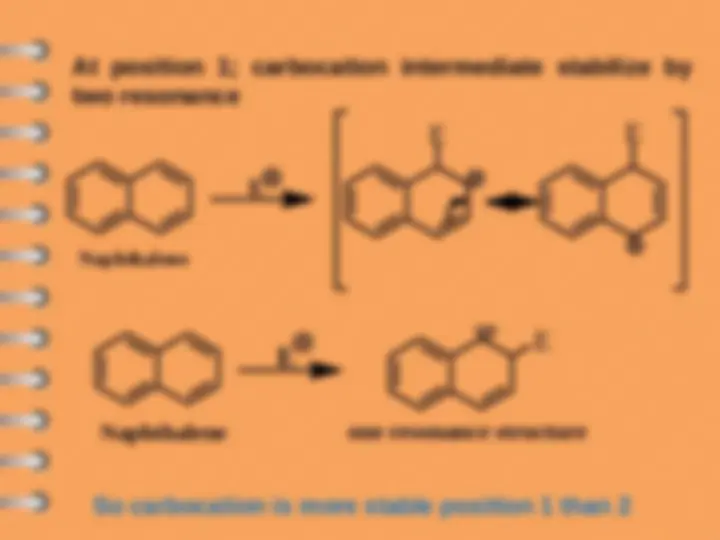
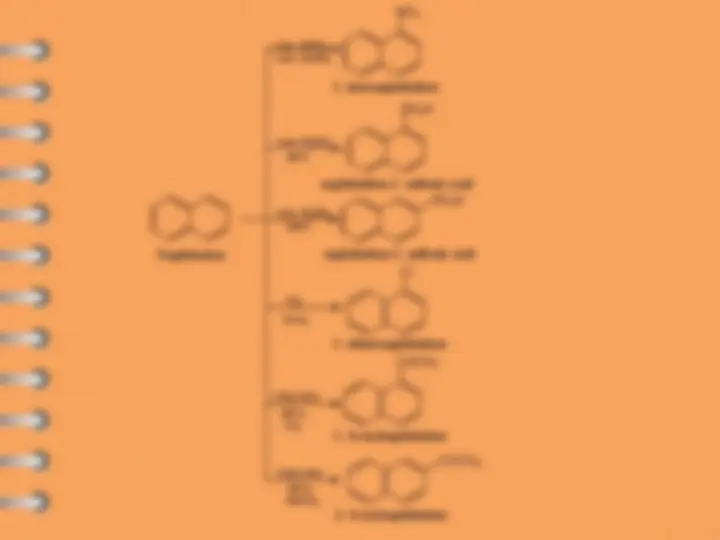
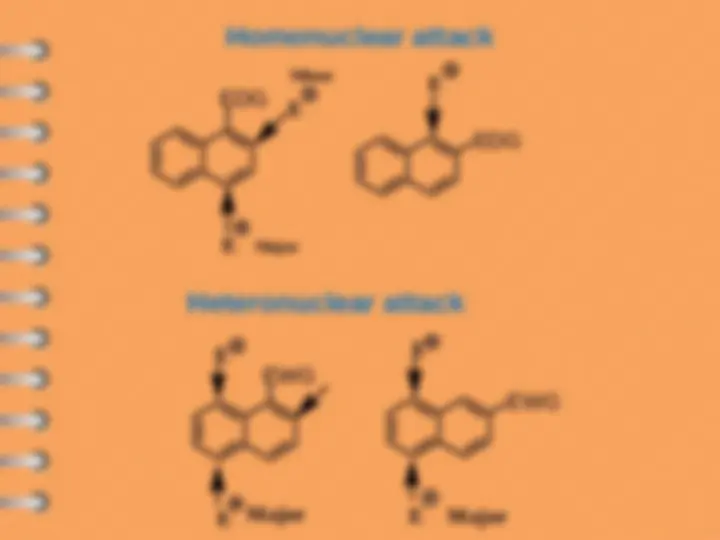
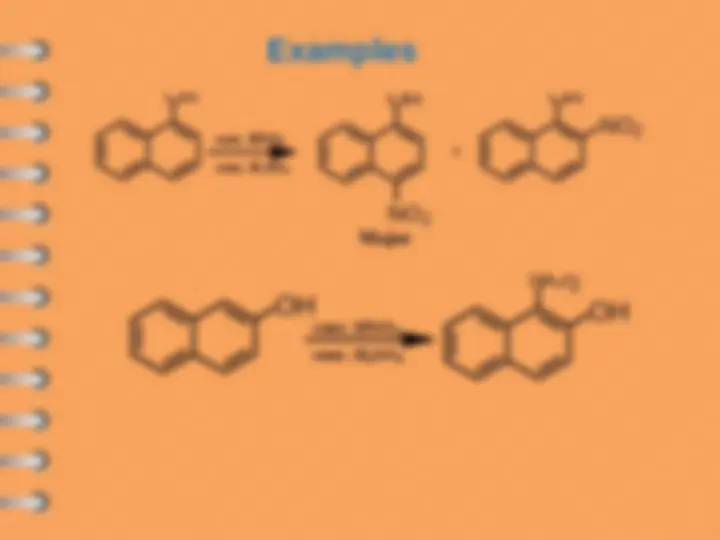
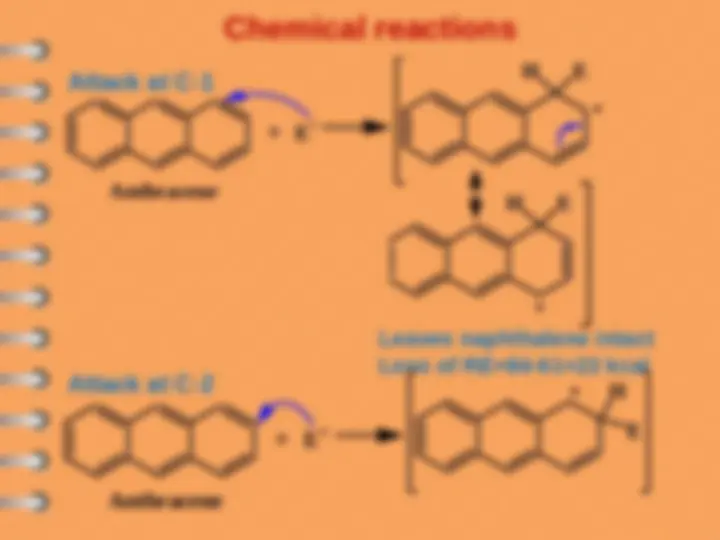
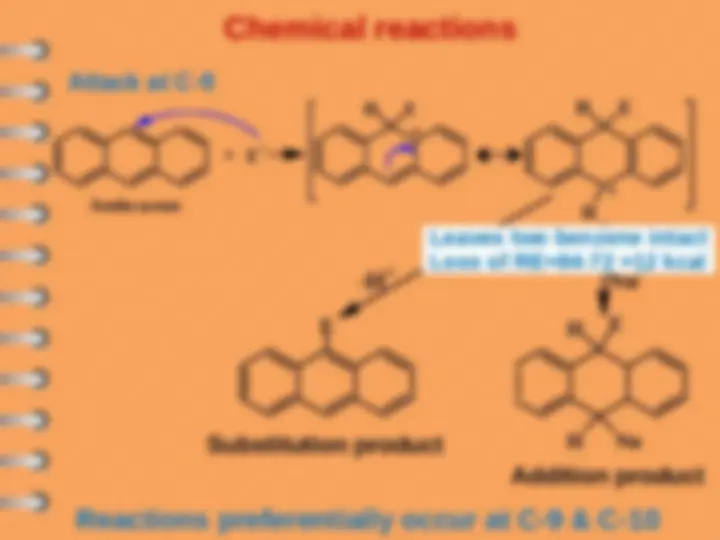
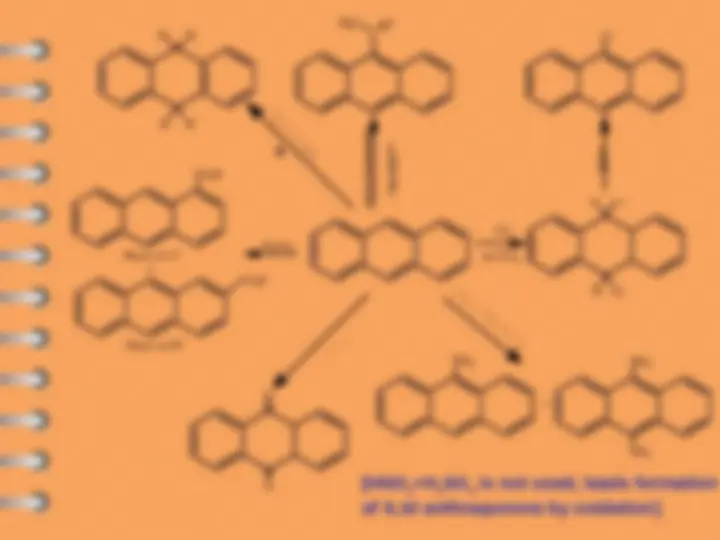
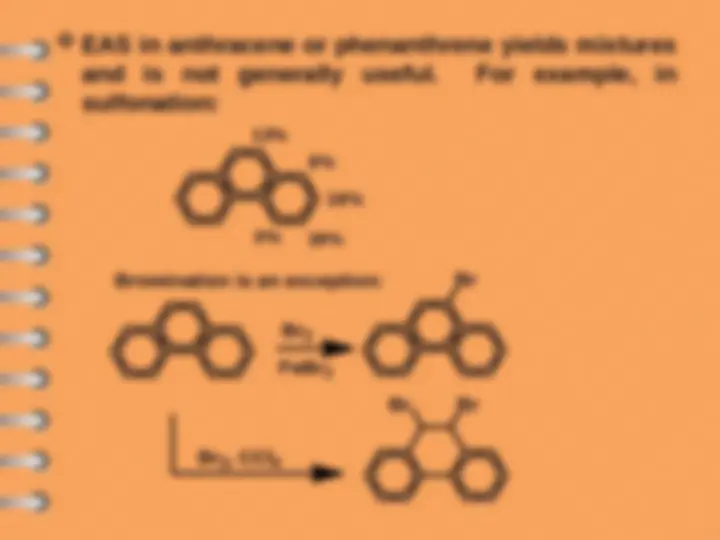
Naphthalene undergoes ES mostly at alpha-position
Attack at C-
Attack at C-
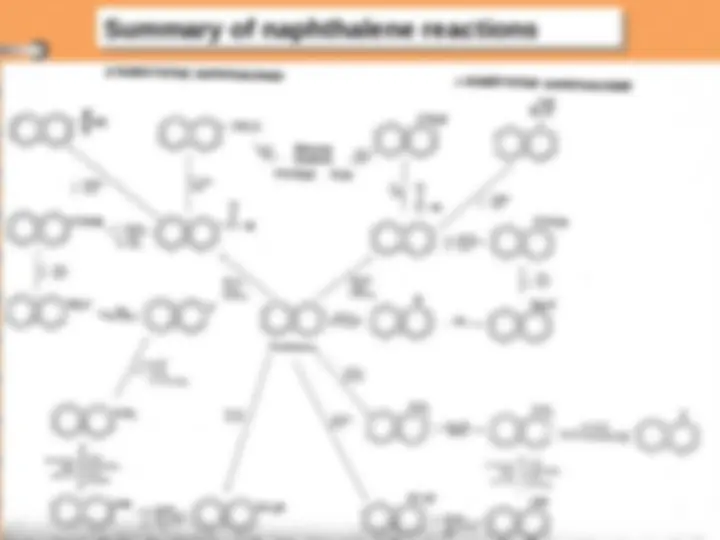
Electrophilic substitution reaction
Naphthalene
naphthalene- 1 - sulfonic acid
Cl
2
SO
3
H
Cl
1 - chloronaphthalene
NO
2
conc. HNO 3
conc. H
2
SO
4
1 - nitronaphthalene
conc. H
2
SO
4
40 ° C
conc. H 2
SO 4
180 ° C
SO
3
H
naphthalene- 2 - sulfonic acid
FeCl
3
CH
3
COCl
AlCl 3
CS 2
COCH
3
1 - Acetylnaphthalene
CH 3
COCl
AlCl 3
PhNO 2
COCH
3
2 - Acetylnaphthalene
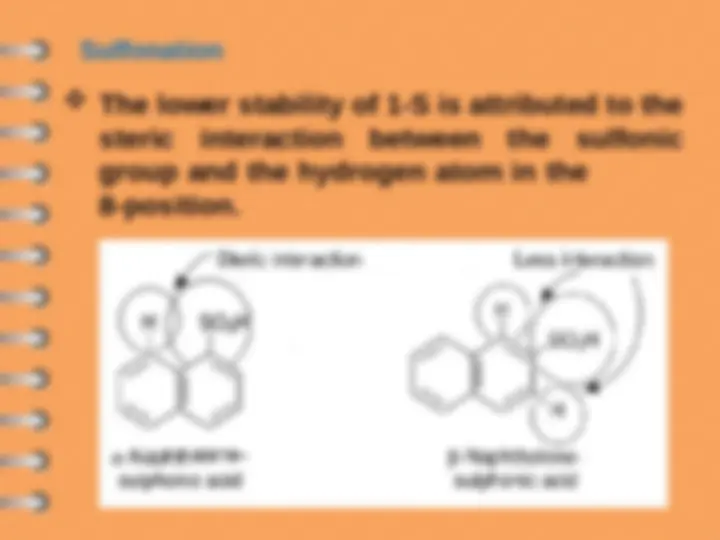
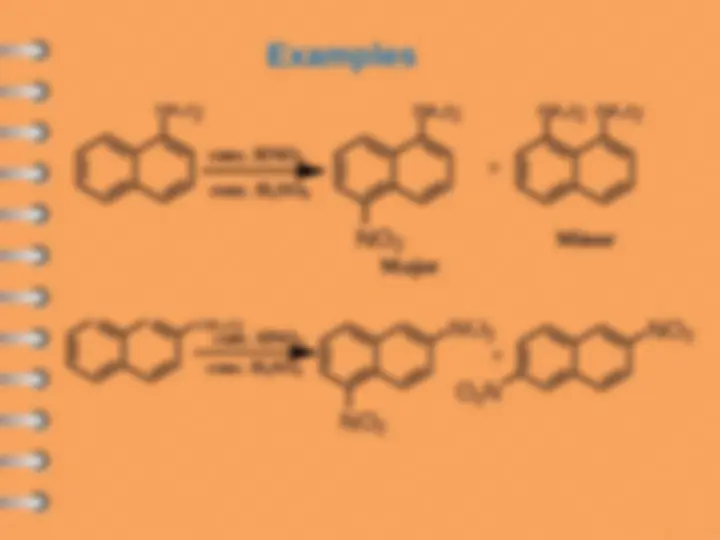
The lower stability of 1-S is attributed to the
steric interaction between the sulfonic
group and the hydrogen atom in the
8-position.
Sulfonation