




Study with the several resources on Docsity

Earn points by helping other students or get them with a premium plan


Prepare for your exams
Study with the several resources on Docsity

Earn points to download
Earn points by helping other students or get them with a premium plan
Community
Ask the community for help and clear up your study doubts
Discover the best universities in your country according to Docsity users
Free resources
Download our free guides on studying techniques, anxiety management strategies, and thesis advice from Docsity tutors
Great schemes about weak acid strong base titration curve
Typology: Schemes and Mind Maps
1 / 6

This page cannot be seen from the preview
Don't miss anything!




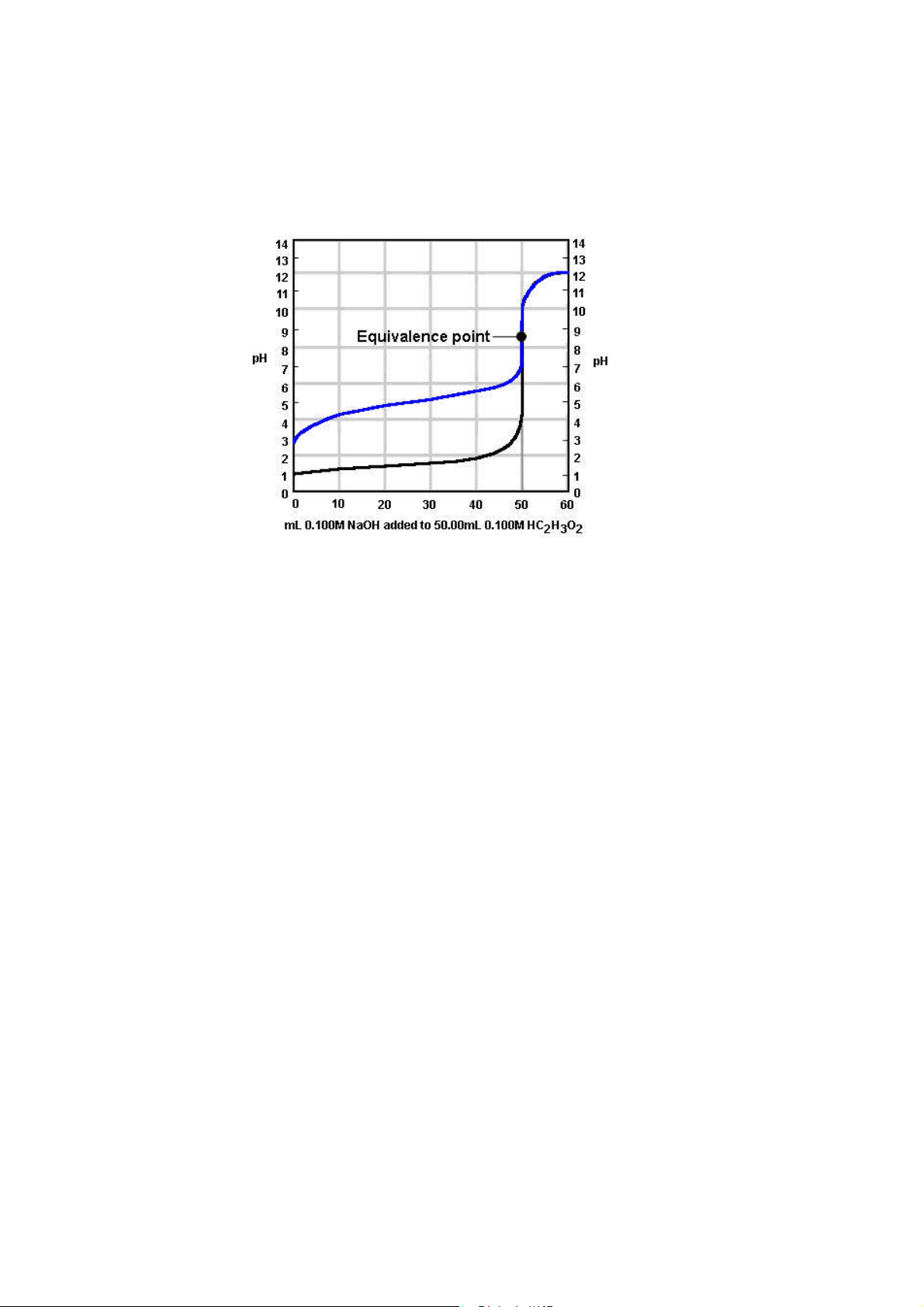
Here, 0.100 M NaOH is being added to 50.0 mL of 0.100 M acetic acid. There are three major differences between this curve (in blue) and the one we saw before (in black):
Titration curve of a weak acid being titrated by a strong base:
EXAMPLE: What is the pH when 30.0 mL of 0.100 M NaOH have been added to 50.0 mL of 0.100 M acetic acid? STEP 1: Stochiometric calculation: The original number of moles of HC 2 H 3 O 2 in the solution is : 50.0 x 10 3 L x 0.100 M = 5.00 x 10 3 moles HC 2 H 3 O 2 Similarly, there are 3.00 x 10 3 moles of OH due to the NaOH solution. The reaction goes to completion: OH (aq) + HC 2 H 3 O 2 (aq) > C 2 H 3 O 2 (aq) + H 2 O (l) The total volume is 80.0 mL. We now calculate the resulting molarities : [HC 2 H 3 O 2 ] = { 2.00 x 10 3 mol HC 2 H 3 O 2 / 0.0800 L } = 0.0250 M [C 2 H 3 O 2 ] = { 3.00 x 10 3 mol C 2 H 3 O 2 } / 0.0800 L } = 0.0375 M STEP 2: Equilibrium calculation, using simplification: Ka = { [H
][C 2 H 3 O 2 ] / [HC 2 H 3 O 2 ] } = 1.8 x 10 5 [H
] = { KA [HC 2 H 3 O 2 ] / [C 2 H 3 O 2 ] } = { (1.8 x 10 5 )(0.0250) / (0.0375) } = 1.2 x 10 5 M pH = log(1.2 x 10 5 ) = 4.
Titrations of Polyprotic Acids An example of a polyprotic acid is H 2 CO 3 which neutralizes in two steps: H 2 CO 3 (aq) + OH (aq) H 2 O (l) + HCO 3 (aq) HCO 3 (aq) + OH (aq) H 2 O (l) + CO 3 2 (aq) The titration curve for these reactions will look like this, with two equivalence points.
Uses of Titration Curves Use titration data or a titration curve to calculate reaction quantities such as the concentration of the substance being titrated. The most common use of titrations is for measuring unknown concentrations. This is done by titrating a known volume of the unknown solution with a solution of known concentration (where the two react in a predictable manner) and finding the volume of titrant needed to reach the equivalence point using some method appropriate to the particular reaction. Then, the volume and concentration of titrant can be used to calculate the moles of titrant added, which, when used with the reaction stoichiometry, gives the number of moles of substance being titrated. Finally, this quantity, along with the volume of substance being titrated, gives the unknown concentration. For acidbase titrations, the equivalence point can be found very easily. A pH meter is simply placed in the solution being titrated and the pH is measured after various volumes of titrant have been added to produce a titration curve. The equivalence point can then be read off the curve.